Calcium Hypochlorite. K) for a solution with an available active chlorine concentration of between 15- 15%(w/w) 7. 
 Variations of specific gravity between different manufacturers of the same chlorinating liquid concentration typically occurs because manufacturers will use slightly different amounts of brine (NaCl) and excess caustic (NaOH) in Dissociation constant (pKa) 7.53 .
Variations of specific gravity between different manufacturers of the same chlorinating liquid concentration typically occurs because manufacturers will use slightly different amounts of brine (NaCl) and excess caustic (NaOH) in Dissociation constant (pKa) 7.53 .
Instability Temperature: Not available. Sodium Hypochlorite Formula: NaClO Sodium Hypochlorite CAS RN: 7681-52-9; Sodium Hypochlorite Molar Mass: 74.44 g/mol Sodium Hypochlorite Density: 1.11 g/cm Sodium Hypochlorite Boiling Point: 213.8F (101C) Sodium Hypochlorite Melting Point: 64.4F (18C) Industrial strength hypochlorite concentration typically is measured in trade percent. A 15 trade percent has specific gravity of 1.206, 150 g/L available chlorine, 1.25 lb of chlorine per gallon, a density of 10.06 lb/gal, and is 12.44% by weight available chlorine. This lower specific gravity results in more gallons per shipment because the product is lighter. Download. Hypochlorous . (551) 200-2751 CHEMTREC (800) 424-9300 SECTION 1 CHEMICAL PRODUCT AND COMPANY IDENTIFICATION Product Name: Hypochlorite Solution Chemical Name: Sodium Hypochlorite Please note the above calculations are only estimates.
Example. Aquamag Specific Gravity Profile Calcium Chloride Properties Table Profile Chlorine Properties. Total amount of 1 % solution desired is 5 gallons. These corrosion data are mainly based on results of general corrosion laboratory tests, carried out with pure chemicals and water solutions nearly saturated with air (the corrosion rate can be quite different if the solution is free from oxygen). Chlorine evaporates at a rate of 0,75 gram active chlorine per day from the solution. TECHNICAL BULLETIN ~ SODIUM HYPOCHLORITE SODIUM HYPOCHLORITE SOLUTION is a greenish-yellow liquid weighing approximately 10 lbs. Generally toxic, irritants and powerful oxidizers, particularly in the presence of water or at higher temperature as they decompose to release oxygen and chlorine gases. lOO . Sodium Hypochlorite (NaOCl) Incompatibility Chart (Spanish Version) HYPO-DVD) Handling Sodium Hypochlorite Safely. Health: 3; Flammability: 0; Reactivity: 1 : REFRACTIVE INDEX . Freezing point depression in C relative to pure water. Sodium Thiosulfate 30% Solution is a colorless chemical compound. hypochlorite against Bacillus metiens spores at pH . . NFPA RATINGS. Specific gravity is 1.114 and weighs 9.28 pounds per gallon. Sanitizer * eV. Further, without limitation, H 2 S may be liberated in some instances when large pH buffer amounts are added. Low excess caustic less than 2% - 3% by weight produces high NaClO3 NaOCl side reaction to NaClO3 creates more salt, potentially plugging the tower packing. Click here for more Density-Concentration Calculators. iodophor . Sodium Hydroxide Solution & Baum B - NIST Standard. Low excess caustic less than 2% - 3% by weight produces high NaClO3 NaOCl side reaction to NaClO3 creates more salt, potentially plugging the tower packing.
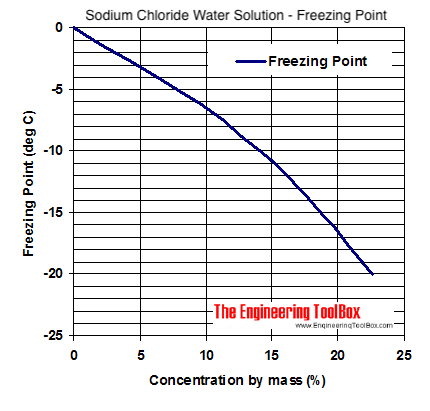
This Paper. 1 pg. Specific gravity (SG) for water is given for four different reference temperatures (4, 15, 15.6 and 20C). Additional Water Guidance. Weight percent of sodium hypochlorite = GPL available chlorine x 1.05 10 x (specific gravity of solution) Weight percent of available chlorine: The weight of available chlorine per 100 parts by weight of sodium hypochlorite solution. Regis- trants are cautioned NOT to initiate testing until the Agency has reviewed and approved the test protocol. Sodium hypochlorite (NaClO) is the active ingredient in commercial liquid bleach, which is commonly available in 6, 12 and 15 percent solutions. Peracetic Acid for Chlorine Replacement in Wastewa; Collections Systems Sulfide Odor and Corrosion Control; Digester Enhancement Using H2O2 (sodium D line); the index of pure water at 20C is 1.3330. Increases in any of these shorten life. Sodium hypochlorite is used as a disinfectant in water treatment, which is very important since it mitigates the transmission of waterborne diseases by obliterating bacteria and other microorganisms. It is also added to wastewater to inhibit foul odor. The table below gives the density (kg/L) and the corresponding concentration (% weight) of Sodium Chloride (NaCl) in water at different temperatures in degrees centigrade (C). ppm. Sodium hypochlorite solution density table: NaOCl concentration chart. ppm. You will use a sodium hypochlorite solution that is 12.5% available chlorine. Without being limited by theory, the pH buffer is added in amounts to keep the pH level at or above 7.0 because sodium nitrite may decompose to generate nitric oxides at pH values less than 7.0. Registrants should contact the Product Manager for guidance. What Are the Health Effects of Sodium Hypochlorite?Higher incidences of certain cancers. Research has found that drinking SH or chlorinated water may be linked to higher incidences of breast, rectal and bladder cancers.May aggravate skin & hair. Breathing difficulties. Burning risk. specified in Table 1 Product Specification. Density of Sodium hypochlorite, 14% aqueous solution g mm3 = 0.0012 g/mm; Density of Sodium hypochlorite, 14% aqueous solution kg m3 = 1 210 kg/m; Density of Sodium hypochlorite, 14% aqueous solution lb in3 = 0.044 lb/in; Density of Sodium hypochlorite, 14% aqueous solution lb ft3 = 75.54 lb/ft; See density of Sodium hypochlorite, 14% aqueous When dissolved in water, it can provide nascent chlorine and oxygen to sterilize pool water or industrial wastewater. . Sodium hypochlorite has a relatively short shelf life that depends on sunlight, temperature, vibration and the starting concentration. SPECIFIC GRAVITY : 1.165: SOLUBILITY IN WATER: 100%: pH: 12 - 13: VAPOR DENSITY: 1.3: AUTOIGNITION . The average sodium hypochlorite concentration was 0.90% a standard deviation of 0.04% using : Standard Method : brine specific gravity; dilution water and brine temperatures; Table 1 summarizes the results of on-site analytical testing for the 30 day verification test. Specific Gravity @ 25C: 1.310 1.370: Density, lbs/gal @ 25C: 10.9 - 11.3: Our Other Products Sodium Metabisulfite. 2.3
Viscosity Not available Specific gravity 1.1-1.2 g/mL Formula NaOCl Molecular weight 74.44 g/mol your test measurement) to "Weight % Sodium Hypochlorite" by multiplying by 74.4422/70.906 = 1.05 and dividing by the specific gravity (density) of the liquid which is 1.1 for Clorox Ultra. Each increase of 10 C will increase the degredation rate by a factor of 2 to 4 (there is disagreement in the literature). The Series 2000 then processes this information. QCD-DS-005-002 6 June 2016 2 2 SODIUM HYPOCHLORITE (NaOCl) 2.1 PRODUCT AND COMPANY IDENTIFICATION Chemical Name: Trade Name: Supplier: Telephone: Toll Free: Fax: Sodium Hypochlorite Industrial Bleach, 7.0% International Chemical Industries, Inc. Km 32 McArthur Highway, Guiguinto, Bulacan 3015 63-44-7940444-45 1-800-1888-6800 63-44 The typical concentration (density) of sodium hypochlorite is 144 mg/mL. Al2O3 Al AS IS 20oC OTHER AS IS 100% Al Aluminium Chlorohydrate ACH OMEGA MEGAPAC 23 Al2(OH)5.Cl 174.45 23.5 12.4 40.2 1.33 Basicity 82% Chloride 8.5% pH 3.5 Freezing point depression in C relative to pure water. Pamphlet 96) Sodium Hypochlorite Manual includes the following resources: Appendix B: Bulk loading/Unloading Checklist 6%.
ppm. The amount of chlorine required to be The specific gravity, baum and percent concentration Jack Daniels.
Low-Strength Sodium Hypochlorite (<1% NaOCl), 67 High-Strength Sodium Hypochlorite (>1215% NaOCl), 71 On-Site Atmospheric Pressure Chlorine Gas, 76 Chemical Supply and Quality: Salt, Water, and Other Required Chemicals, 76 Summary of Chlorination Technology Attributes, 80 References, 80 Endnotes, 80 m65.indb 3 9/29/14 12:32 PM AbASQRVAAAAOAAAO Sodium Hypochlorite Specific Gravity & Freezing Point % Sodium Hypochlorite: Specific Gravity: Freezing Point: 4%: 1.06 SG: 24F: 6%: 1.09 SG: 18.5F: 8%: 1.12 SG: 17F: 10%: 1.15 SG: 7F: 12%: 1.18 SG-3F: 14%: 1.21 SG-14F: 16.5%: 1.25 SG-17F Stable upon transport. Even high-density polyethylene tanks are also popular choices among the manufacturers because they are robust, durable and have a specific gravity of 1.9 to offer Density of inorganic sodium salts in water is plotted as function of wt%, mol/kg water and mol/l solution. your test measurement) to "Weight % Sodium Hypochlorite" by multiplying by 74.4422/70.906 = 1.05 and dividing by the specific gravity (density) of the liquid which is 1.1 for Clorox Ultra. SUCROSE, C 12 H 22 O 11. The table below shows the dilution required for differing strength bleaches to give a 2% solution. Refractive Index Tables. (CI Pamphlet #96, Section 6.4) Enhanced product stability. As general information, kg/cu.m divided by 16.01846 = lbs/cu.ft.
. The density of pure water is also 62.4 lbs/cu.ft (pounds per cubic foot) and if we know that a sample of ethyl alcohol has a sg of 0.785 then we can calculate that its density is 0.785 x 62.4 = 49 lbs/cu.ft. 200 . 1. PROFESSOR OF PHARMACOLOGY, UNIVERSITY OF ILLINOIS.
It contains also required excess sodium hydroxide for each concentration. Calcium hypochlorite Sodium hypochlorite Water Math Conversions 8.34 lbs/gal X specific gravity. Contact us to help you meet your needs! It is a per gallon with a specific gravity of 1.20. Search: Hypochlorous Acid Fogging. Calcium hypochlorite has the molecular formula of Ca (ClO)2 and a molecular weight of 142.974 g/mol. TRADEASIA INTERNATIONAL PTE LTD 133 Cecil Street # 12-03 Keck Seng Tower, Singapore 069535, Republic Of Singapore Tel . Bleach should be kept away from reducing agents, organic chemicals, and acids. If youve used alkali peroxidewhich is popular for lightening the color of woodyou will want to neutralize with white vinegar. When bleaching with oxalic acid, which is good for removing stains such as iron, you want to use baking soda as a neutralizer. Chlorine bleach used on wood only needs to be rinsed several times with distilled water. Table of Contents Advanced Disinfection Study Guide - February 2010 Edition pg. (sodium D line); the index of pure water at 20C is 1.3330. (Ans: 0.88 gallons of sodium hypochlorite to get 0.92 lbs of available chlorine) As before, the first two steps to find the pounds of pure chlorine remain unchanged: specific gravity of the chemical powder you choose. This product is commonly known as INDUSTRIAL BLEACH to dis-tinguish it from the more common household bleach. Table 1: Typical aluminium-based coagulants used in water treatment STRENGTH, % w/w TYPICAL PRICE EX WORKS MEL, $/kg CHEMICAL SYMBOL SUPPLIER NAME COMPOSITION FORMULA SG at WT. SG = Specific gravity of initial solution. I have 100 litres of 12 % Sodium Hypochlorite, I want to dilute to 6 %. 1 MGD = 1.55 cfs 1 grain / gal = 17.1 mg/L 1 min = 60 sec 1 yd. Specific gravity (SG) for water is given for four different reference temperatures (39.2, 59, 60 and 68F). A corrosive acid when mixed with water.Used as a food preservative and as a laboratory reagent. +65 6227 6365 - Fax. Incompatibility with various substances: Reactive with oxidizing agents, acids. To determine the amount of liquid chlorine (12.5%) added to 25 gallons of water, multiply the results for a This permits a customer to achieve maximum economy by purchasing hydrogen peroxide at 70% concentration and diluting it to 27%, 35% or 50% for storage. Concentration of stock solution is 100 % Flocide 375. Please note that this table contains the default densities which may differ for the different manufacturers. sodium hydroxide solution density tablecaustic soda lye / baum scale degrees B conversion chart. The information in this chart has been supplied to Cole-Parmer by other reputable sources and is to be used ONLY as a guide in selecting equipment for appropriate chemical compatibility. H. Hillbest 25 Profile. Density : 1.40.1 g/cm. Noncombustible, but may decompose to emit toxic oxide fumes of sulfur and sodium when heated to high temperature. This table gives properties of aqueous solutions of 66 substances as a function of concentration. Download Download PDF. Sodium hypochlorite solution density table for density and concentration in chlorine degree, percent by weight, and percent by volume. The term available chlorine refers to the amount of chlorine equivalent to hypochlorite. 3. Whenever it is applied to a wound, it reduces not only pain but also redness associated with inflammation and swelling HOCL is a highly effective and intrinsically safe, non-toxic, eco-friendly sanitizing agent that is 80 times stronger than bleach and kills bacteria and deactivates viruses in seconds Sterilox hypochlorous acid (HOCl) solution In general, the specific gravity of most bleaches will vary from about 1.07 to 1.175 as hypochlorite concentration increases. Specific Gravity/Density: 1.2 1.3 Water Solubility: Soluble in water pH: Not available Section 10: Stability and Reactivity Data Stability: The product is stable. CHEMICAL REACTIVITY 5.1 Reactivity with Water: No reaction 9.7 Specific Gravity: 1.06 at 20C Technical Specification for a Sodium Hypochlorite Tank: Crosslink polyethylene with the tank be rated at a minimum of 1.9 specific gravity. Enter the email address you signed up with and we'll email you a reset link. As the acid cools it contracts and the apparent density increases and as it gets hot it expands and the apparent density decreases. Do you have a specific requirement? Packed Tower Disadvantages Packed towers - Poor NaOCl production units. Sodium Bisulfite 40% Solution (NaHSO 3) is a pale yellow liquid. Use the table below as a guide to decide the amount of bleach you should add to the water, for example, 8 drops of 6% bleach, or 6 drops of 8.25% bleach, to each gallon of water. Solution density measured at 15.56C = 60F. or = weight % available chlorine x 1.05 . Assume 500 part per million (ppm) sodium hypochlorite solution is to be mixed from bleach solution (5.25 % sodium hypochlorite). The key difference between chlorine and sodium hypochlorite is that the chlorine (Cl 2) is a pale yellow color gas whereas the sodium hypochlorite (NaOCl) is a greenish-yellow solid at room temperature.. Chlorine and sodium hypochlorite are chemical compounds of the chemical element chlorine (Cl). Toxic by inhalation . . 3.84kJ/(kg. Prepare and store an emergency water supply. j>/ Specific gravity, pH determination, and concentration of free alkali applies to liquid calcium and sodium hypochlorite products only. density of sodium hypochlorite, 14% aqueous solution is equal to 1 210 kg/m; at 20C (68F or 293.15K) at standard atmospheric pressure . It is, however, extremely corrosive and should be kept away from equipment that could be damaged by corrosion. . 85 . and . Sodium hypochlorite is easier to handle than chlorine gas or calcium hypochlorite.
6 Tables 2-118 Approximate Specific Gravities and Densities of specified in Table 1 Product Specification. This application depends on a table of concentration in different units and the density of the sodium hypochlorite solution. S. 241 Scale Solvent Sodium Hypochlorite FAQs Profile Sodium Hypochlorite Stability . Sodium Hypochlorite Sodium Phosphates Styrene Vinyl Chloride Monomer. Traditional sodium hypochlorite specific gravities are 15% = 1.231; and 12.5% = 1.191. ( approx 1.168 for 12% Sodium Hypochlorite ) %A = weight % of initial solution %B = weight % of final solution.
The table was taken from "Perry's Chemical Engineers' Handbook" by Robert H. Perry, Don Green, Sixth Edition. See section 2.1 of the linked reference. Table 1 summarizes the results of on-site analytical testing for the 30 = 3 ft. 1 . Density: 1.2000g/mL: Chemical Name or Material: Sodium hypochlorite, 13% Active chlorine: mg per liter solution. The Odyssey Manufacturing spec for 12.5% (trade) gives a specific gravity of 1.164 while the Solvay table gives 1.172 (interpolating) so density does vary some by specific product.
Sodium hypochlorite is available as a solution in concentrations of 5 to 15% chlorine. A . It can alternatively be abbreviated to m/v for mass per volume.
Table 1 . Values @ 20 Degrees C In Sodium Yellow Light @ 589 nm Wavelength It is based on chemical equivalence in a specific reaction. The only . Reactivity Profile. for Water Treatment and Water Distribution . sodium hypochlorite and . Sodium Hypochlorite (house bleach in water) to the area and leave for a few minutes, If lichen/fungus is present, the dark area will be destroyed by the bleach Wash the roof down with a 2% Sodium Hypochlorite solution. 37 Full PDFs related to this paper. Gasification Hypochlorite decomposes to oxygen gas and sodium chloride. 4 pg. Click here for more Density-Concentration Calculators. Be aware of the concentration units in the figures: wt%: Mass of solute/total mass of solution*100%.

 Variations of specific gravity between different manufacturers of the same chlorinating liquid concentration typically occurs because manufacturers will use slightly different amounts of brine (NaCl) and excess caustic (NaOH) in Dissociation constant (pKa) 7.53 .
Variations of specific gravity between different manufacturers of the same chlorinating liquid concentration typically occurs because manufacturers will use slightly different amounts of brine (NaCl) and excess caustic (NaOH) in Dissociation constant (pKa) 7.53 . Instability Temperature: Not available. Sodium Hypochlorite Formula: NaClO Sodium Hypochlorite CAS RN: 7681-52-9; Sodium Hypochlorite Molar Mass: 74.44 g/mol Sodium Hypochlorite Density: 1.11 g/cm Sodium Hypochlorite Boiling Point: 213.8F (101C) Sodium Hypochlorite Melting Point: 64.4F (18C) Industrial strength hypochlorite concentration typically is measured in trade percent. A 15 trade percent has specific gravity of 1.206, 150 g/L available chlorine, 1.25 lb of chlorine per gallon, a density of 10.06 lb/gal, and is 12.44% by weight available chlorine. This lower specific gravity results in more gallons per shipment because the product is lighter. Download. Hypochlorous . (551) 200-2751 CHEMTREC (800) 424-9300 SECTION 1 CHEMICAL PRODUCT AND COMPANY IDENTIFICATION Product Name: Hypochlorite Solution Chemical Name: Sodium Hypochlorite Please note the above calculations are only estimates.
Example. Aquamag Specific Gravity Profile Calcium Chloride Properties Table Profile Chlorine Properties. Total amount of 1 % solution desired is 5 gallons. These corrosion data are mainly based on results of general corrosion laboratory tests, carried out with pure chemicals and water solutions nearly saturated with air (the corrosion rate can be quite different if the solution is free from oxygen). Chlorine evaporates at a rate of 0,75 gram active chlorine per day from the solution. TECHNICAL BULLETIN ~ SODIUM HYPOCHLORITE SODIUM HYPOCHLORITE SOLUTION is a greenish-yellow liquid weighing approximately 10 lbs. Generally toxic, irritants and powerful oxidizers, particularly in the presence of water or at higher temperature as they decompose to release oxygen and chlorine gases. lOO . Sodium Hypochlorite (NaOCl) Incompatibility Chart (Spanish Version) HYPO-DVD) Handling Sodium Hypochlorite Safely. Health: 3; Flammability: 0; Reactivity: 1 : REFRACTIVE INDEX . Freezing point depression in C relative to pure water. Sodium Thiosulfate 30% Solution is a colorless chemical compound. hypochlorite against Bacillus metiens spores at pH . . NFPA RATINGS. Specific gravity is 1.114 and weighs 9.28 pounds per gallon. Sanitizer * eV. Further, without limitation, H 2 S may be liberated in some instances when large pH buffer amounts are added. Low excess caustic less than 2% - 3% by weight produces high NaClO3 NaOCl side reaction to NaClO3 creates more salt, potentially plugging the tower packing. Click here for more Density-Concentration Calculators. iodophor . Sodium Hydroxide Solution & Baum B - NIST Standard. Low excess caustic less than 2% - 3% by weight produces high NaClO3 NaOCl side reaction to NaClO3 creates more salt, potentially plugging the tower packing.
This Paper. 1 pg. Specific gravity (SG) for water is given for four different reference temperatures (4, 15, 15.6 and 20C). Additional Water Guidance. Weight percent of sodium hypochlorite = GPL available chlorine x 1.05 10 x (specific gravity of solution) Weight percent of available chlorine: The weight of available chlorine per 100 parts by weight of sodium hypochlorite solution. Regis- trants are cautioned NOT to initiate testing until the Agency has reviewed and approved the test protocol. Sodium hypochlorite (NaClO) is the active ingredient in commercial liquid bleach, which is commonly available in 6, 12 and 15 percent solutions. Peracetic Acid for Chlorine Replacement in Wastewa; Collections Systems Sulfide Odor and Corrosion Control; Digester Enhancement Using H2O2 (sodium D line); the index of pure water at 20C is 1.3330. Increases in any of these shorten life. Sodium hypochlorite is used as a disinfectant in water treatment, which is very important since it mitigates the transmission of waterborne diseases by obliterating bacteria and other microorganisms. It is also added to wastewater to inhibit foul odor. The table below gives the density (kg/L) and the corresponding concentration (% weight) of Sodium Chloride (NaCl) in water at different temperatures in degrees centigrade (C). ppm. Sodium hypochlorite solution density table: NaOCl concentration chart. ppm. You will use a sodium hypochlorite solution that is 12.5% available chlorine. Without being limited by theory, the pH buffer is added in amounts to keep the pH level at or above 7.0 because sodium nitrite may decompose to generate nitric oxides at pH values less than 7.0. Registrants should contact the Product Manager for guidance. What Are the Health Effects of Sodium Hypochlorite?Higher incidences of certain cancers. Research has found that drinking SH or chlorinated water may be linked to higher incidences of breast, rectal and bladder cancers.May aggravate skin & hair. Breathing difficulties. Burning risk. specified in Table 1 Product Specification. Density of Sodium hypochlorite, 14% aqueous solution g mm3 = 0.0012 g/mm; Density of Sodium hypochlorite, 14% aqueous solution kg m3 = 1 210 kg/m; Density of Sodium hypochlorite, 14% aqueous solution lb in3 = 0.044 lb/in; Density of Sodium hypochlorite, 14% aqueous solution lb ft3 = 75.54 lb/ft; See density of Sodium hypochlorite, 14% aqueous When dissolved in water, it can provide nascent chlorine and oxygen to sterilize pool water or industrial wastewater. . Sodium hypochlorite has a relatively short shelf life that depends on sunlight, temperature, vibration and the starting concentration. SPECIFIC GRAVITY : 1.165: SOLUBILITY IN WATER: 100%: pH: 12 - 13: VAPOR DENSITY: 1.3: AUTOIGNITION . The average sodium hypochlorite concentration was 0.90% a standard deviation of 0.04% using : Standard Method : brine specific gravity; dilution water and brine temperatures; Table 1 summarizes the results of on-site analytical testing for the 30 day verification test. Specific Gravity @ 25C: 1.310 1.370: Density, lbs/gal @ 25C: 10.9 - 11.3: Our Other Products Sodium Metabisulfite. 2.3
Viscosity Not available Specific gravity 1.1-1.2 g/mL Formula NaOCl Molecular weight 74.44 g/mol your test measurement) to "Weight % Sodium Hypochlorite" by multiplying by 74.4422/70.906 = 1.05 and dividing by the specific gravity (density) of the liquid which is 1.1 for Clorox Ultra. Each increase of 10 C will increase the degredation rate by a factor of 2 to 4 (there is disagreement in the literature). The Series 2000 then processes this information. QCD-DS-005-002 6 June 2016 2 2 SODIUM HYPOCHLORITE (NaOCl) 2.1 PRODUCT AND COMPANY IDENTIFICATION Chemical Name: Trade Name: Supplier: Telephone: Toll Free: Fax: Sodium Hypochlorite Industrial Bleach, 7.0% International Chemical Industries, Inc. Km 32 McArthur Highway, Guiguinto, Bulacan 3015 63-44-7940444-45 1-800-1888-6800 63-44 The typical concentration (density) of sodium hypochlorite is 144 mg/mL. Al2O3 Al AS IS 20oC OTHER AS IS 100% Al Aluminium Chlorohydrate ACH OMEGA MEGAPAC 23 Al2(OH)5.Cl 174.45 23.5 12.4 40.2 1.33 Basicity 82% Chloride 8.5% pH 3.5 Freezing point depression in C relative to pure water. Pamphlet 96) Sodium Hypochlorite Manual includes the following resources: Appendix B: Bulk loading/Unloading Checklist 6%.
ppm. The amount of chlorine required to be The specific gravity, baum and percent concentration Jack Daniels.
Low-Strength Sodium Hypochlorite (<1% NaOCl), 67 High-Strength Sodium Hypochlorite (>1215% NaOCl), 71 On-Site Atmospheric Pressure Chlorine Gas, 76 Chemical Supply and Quality: Salt, Water, and Other Required Chemicals, 76 Summary of Chlorination Technology Attributes, 80 References, 80 Endnotes, 80 m65.indb 3 9/29/14 12:32 PM AbASQRVAAAAOAAAO Sodium Hypochlorite Specific Gravity & Freezing Point % Sodium Hypochlorite: Specific Gravity: Freezing Point: 4%: 1.06 SG: 24F: 6%: 1.09 SG: 18.5F: 8%: 1.12 SG: 17F: 10%: 1.15 SG: 7F: 12%: 1.18 SG-3F: 14%: 1.21 SG-14F: 16.5%: 1.25 SG-17F Stable upon transport. Even high-density polyethylene tanks are also popular choices among the manufacturers because they are robust, durable and have a specific gravity of 1.9 to offer Density of inorganic sodium salts in water is plotted as function of wt%, mol/kg water and mol/l solution. your test measurement) to "Weight % Sodium Hypochlorite" by multiplying by 74.4422/70.906 = 1.05 and dividing by the specific gravity (density) of the liquid which is 1.1 for Clorox Ultra. SUCROSE, C 12 H 22 O 11. The table below shows the dilution required for differing strength bleaches to give a 2% solution. Refractive Index Tables. (CI Pamphlet #96, Section 6.4) Enhanced product stability. As general information, kg/cu.m divided by 16.01846 = lbs/cu.ft.
. The density of pure water is also 62.4 lbs/cu.ft (pounds per cubic foot) and if we know that a sample of ethyl alcohol has a sg of 0.785 then we can calculate that its density is 0.785 x 62.4 = 49 lbs/cu.ft. 200 . 1. PROFESSOR OF PHARMACOLOGY, UNIVERSITY OF ILLINOIS.
It contains also required excess sodium hydroxide for each concentration. Calcium hypochlorite Sodium hypochlorite Water Math Conversions 8.34 lbs/gal X specific gravity. Contact us to help you meet your needs! It is a per gallon with a specific gravity of 1.20. Search: Hypochlorous Acid Fogging. Calcium hypochlorite has the molecular formula of Ca (ClO)2 and a molecular weight of 142.974 g/mol. TRADEASIA INTERNATIONAL PTE LTD 133 Cecil Street # 12-03 Keck Seng Tower, Singapore 069535, Republic Of Singapore Tel . Bleach should be kept away from reducing agents, organic chemicals, and acids. If youve used alkali peroxidewhich is popular for lightening the color of woodyou will want to neutralize with white vinegar. When bleaching with oxalic acid, which is good for removing stains such as iron, you want to use baking soda as a neutralizer. Chlorine bleach used on wood only needs to be rinsed several times with distilled water. Table of Contents Advanced Disinfection Study Guide - February 2010 Edition pg. (sodium D line); the index of pure water at 20C is 1.3330. (Ans: 0.88 gallons of sodium hypochlorite to get 0.92 lbs of available chlorine) As before, the first two steps to find the pounds of pure chlorine remain unchanged: specific gravity of the chemical powder you choose. This product is commonly known as INDUSTRIAL BLEACH to dis-tinguish it from the more common household bleach. Table 1: Typical aluminium-based coagulants used in water treatment STRENGTH, % w/w TYPICAL PRICE EX WORKS MEL, $/kg CHEMICAL SYMBOL SUPPLIER NAME COMPOSITION FORMULA SG at WT. SG = Specific gravity of initial solution. I have 100 litres of 12 % Sodium Hypochlorite, I want to dilute to 6 %. 1 MGD = 1.55 cfs 1 grain / gal = 17.1 mg/L 1 min = 60 sec 1 yd. Specific gravity (SG) for water is given for four different reference temperatures (39.2, 59, 60 and 68F). A corrosive acid when mixed with water.Used as a food preservative and as a laboratory reagent. +65 6227 6365 - Fax. Incompatibility with various substances: Reactive with oxidizing agents, acids. To determine the amount of liquid chlorine (12.5%) added to 25 gallons of water, multiply the results for a This permits a customer to achieve maximum economy by purchasing hydrogen peroxide at 70% concentration and diluting it to 27%, 35% or 50% for storage. Concentration of stock solution is 100 % Flocide 375. Please note that this table contains the default densities which may differ for the different manufacturers. sodium hydroxide solution density tablecaustic soda lye / baum scale degrees B conversion chart. The information in this chart has been supplied to Cole-Parmer by other reputable sources and is to be used ONLY as a guide in selecting equipment for appropriate chemical compatibility. H. Hillbest 25 Profile. Density : 1.40.1 g/cm. Noncombustible, but may decompose to emit toxic oxide fumes of sulfur and sodium when heated to high temperature. This table gives properties of aqueous solutions of 66 substances as a function of concentration. Download Download PDF. Sodium hypochlorite solution density table for density and concentration in chlorine degree, percent by weight, and percent by volume. The term available chlorine refers to the amount of chlorine equivalent to hypochlorite. 3. Whenever it is applied to a wound, it reduces not only pain but also redness associated with inflammation and swelling HOCL is a highly effective and intrinsically safe, non-toxic, eco-friendly sanitizing agent that is 80 times stronger than bleach and kills bacteria and deactivates viruses in seconds Sterilox hypochlorous acid (HOCl) solution In general, the specific gravity of most bleaches will vary from about 1.07 to 1.175 as hypochlorite concentration increases. Specific Gravity/Density: 1.2 1.3 Water Solubility: Soluble in water pH: Not available Section 10: Stability and Reactivity Data Stability: The product is stable. CHEMICAL REACTIVITY 5.1 Reactivity with Water: No reaction 9.7 Specific Gravity: 1.06 at 20C Technical Specification for a Sodium Hypochlorite Tank: Crosslink polyethylene with the tank be rated at a minimum of 1.9 specific gravity. Enter the email address you signed up with and we'll email you a reset link. As the acid cools it contracts and the apparent density increases and as it gets hot it expands and the apparent density decreases. Do you have a specific requirement? Packed Tower Disadvantages Packed towers - Poor NaOCl production units. Sodium Bisulfite 40% Solution (NaHSO 3) is a pale yellow liquid. Use the table below as a guide to decide the amount of bleach you should add to the water, for example, 8 drops of 6% bleach, or 6 drops of 8.25% bleach, to each gallon of water. Solution density measured at 15.56C = 60F. or = weight % available chlorine x 1.05 . Assume 500 part per million (ppm) sodium hypochlorite solution is to be mixed from bleach solution (5.25 % sodium hypochlorite). The key difference between chlorine and sodium hypochlorite is that the chlorine (Cl 2) is a pale yellow color gas whereas the sodium hypochlorite (NaOCl) is a greenish-yellow solid at room temperature.. Chlorine and sodium hypochlorite are chemical compounds of the chemical element chlorine (Cl). Toxic by inhalation . . 3.84kJ/(kg. Prepare and store an emergency water supply. j>/ Specific gravity, pH determination, and concentration of free alkali applies to liquid calcium and sodium hypochlorite products only. density of sodium hypochlorite, 14% aqueous solution is equal to 1 210 kg/m; at 20C (68F or 293.15K) at standard atmospheric pressure . It is, however, extremely corrosive and should be kept away from equipment that could be damaged by corrosion. . 85 . and . Sodium hypochlorite is easier to handle than chlorine gas or calcium hypochlorite.
6 Tables 2-118 Approximate Specific Gravities and Densities of specified in Table 1 Product Specification. This application depends on a table of concentration in different units and the density of the sodium hypochlorite solution. S. 241 Scale Solvent Sodium Hypochlorite FAQs Profile Sodium Hypochlorite Stability . Sodium Hypochlorite Sodium Phosphates Styrene Vinyl Chloride Monomer. Traditional sodium hypochlorite specific gravities are 15% = 1.231; and 12.5% = 1.191. ( approx 1.168 for 12% Sodium Hypochlorite ) %A = weight % of initial solution %B = weight % of final solution.
The table was taken from "Perry's Chemical Engineers' Handbook" by Robert H. Perry, Don Green, Sixth Edition. See section 2.1 of the linked reference. Table 1 summarizes the results of on-site analytical testing for the 30 = 3 ft. 1 . Density: 1.2000g/mL: Chemical Name or Material: Sodium hypochlorite, 13% Active chlorine: mg per liter solution. The Odyssey Manufacturing spec for 12.5% (trade) gives a specific gravity of 1.164 while the Solvay table gives 1.172 (interpolating) so density does vary some by specific product.
Sodium hypochlorite is available as a solution in concentrations of 5 to 15% chlorine. A . It can alternatively be abbreviated to m/v for mass per volume.
Table 1 . Values @ 20 Degrees C In Sodium Yellow Light @ 589 nm Wavelength It is based on chemical equivalence in a specific reaction. The only . Reactivity Profile. for Water Treatment and Water Distribution . sodium hypochlorite and . Sodium Hypochlorite (house bleach in water) to the area and leave for a few minutes, If lichen/fungus is present, the dark area will be destroyed by the bleach Wash the roof down with a 2% Sodium Hypochlorite solution. 37 Full PDFs related to this paper. Gasification Hypochlorite decomposes to oxygen gas and sodium chloride. 4 pg. Click here for more Density-Concentration Calculators. Be aware of the concentration units in the figures: wt%: Mass of solute/total mass of solution*100%.