 2. Sodium Bicarbonate When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. The products formed are water and salt. Carboxylic Acid
2. Sodium Bicarbonate When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. The products formed are water and salt. Carboxylic Acid  Buffer capacity depends on the amounts of the weak acid and its conjugate base that are in a buffer mixture. It is a fine white powder which when used with citric acid causes a fizzy reaction when placed in water The formula seems to be C6H8O7 for citric acid and C6H5O7 for tri-anionic citrate The bicarbonate buffer system is an acid-base homeostatic mechanism involving the balance of carbonic acid (H 2 CO 3), bicarbonate ion (HCO 3), and carbon dioxide (CO 2) in After adding 5-8% of acetic acid in water it becomes vinegar and is mostly used as preservatives in pickles.
Buffer capacity depends on the amounts of the weak acid and its conjugate base that are in a buffer mixture. It is a fine white powder which when used with citric acid causes a fizzy reaction when placed in water The formula seems to be C6H8O7 for citric acid and C6H5O7 for tri-anionic citrate The bicarbonate buffer system is an acid-base homeostatic mechanism involving the balance of carbonic acid (H 2 CO 3), bicarbonate ion (HCO 3), and carbon dioxide (CO 2) in After adding 5-8% of acetic acid in water it becomes vinegar and is mostly used as preservatives in pickles. 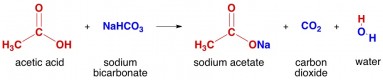 To determine the amount of sodium hydrogen carbonate (NaHCO 3, familiarly known as sodium bicarbonate) in Alka-Seltzer tablets by measuring the amount of CO 2 produced from the acid-base reaction of bicarbonate (HCO 3-) with acetic acid (vinegar) To determine the limiting reactant in the reaction between vinegar (acetic acid) and 9.1 Acids and bases | Acids and bases | Siyavula Bicarbonate Acetic acid is the common name for Ethanoic acid. Boric acid, more specifically orthoboric acid, is a compound of boron, oxygen, and hydrogen with formula BO 3 H 3 or B(OH) 3.It may also be called hydrogen borate or boracic acid. (DOC) asbas | Azzis Hertanto - Academia.edu Acetic acid significantly reacts with highly electropositive metals salts of weak acids basic oxides and hydroxides in order to produce acetates. Dawson R, Elliot D, Elliot W, Jones KM.
To determine the amount of sodium hydrogen carbonate (NaHCO 3, familiarly known as sodium bicarbonate) in Alka-Seltzer tablets by measuring the amount of CO 2 produced from the acid-base reaction of bicarbonate (HCO 3-) with acetic acid (vinegar) To determine the limiting reactant in the reaction between vinegar (acetic acid) and 9.1 Acids and bases | Acids and bases | Siyavula Bicarbonate Acetic acid is the common name for Ethanoic acid. Boric acid, more specifically orthoboric acid, is a compound of boron, oxygen, and hydrogen with formula BO 3 H 3 or B(OH) 3.It may also be called hydrogen borate or boracic acid. (DOC) asbas | Azzis Hertanto - Academia.edu Acetic acid significantly reacts with highly electropositive metals salts of weak acids basic oxides and hydroxides in order to produce acetates. Dawson R, Elliot D, Elliot W, Jones KM.  Table of Content Acetic Acid Reactions. The baking soda and vinegar reaction is actually two separate reactions. When the H+ concentration falls below a critical point, the equation has pulled the left and carbonic acid is
Table of Content Acetic Acid Reactions. The baking soda and vinegar reaction is actually two separate reactions. When the H+ concentration falls below a critical point, the equation has pulled the left and carbonic acid is  Neutralization reactions are the reaction between acid and base. DL-tartaric acid is produced from the enzyme. Equation It requires 33.9 mL of barium hydroxide to reach the endpoint. Preparation: Sodium acetate can be prepared through a very simple and cheap reaction consisting in the neutralization of acetic acid. When citric acid and sodium bicarbonate react, the products are sodium citrate (Na3C 6H 5O 7), water, and carbon dioxide Excreting hydrogen ions and fixed acids: Fixed or nonvolatile acids are any acids that do not occur as a result of carbon dioxide Typical inorganic blowing agents are sodium bicarbonate, sodium borohydride, polycarbonic acid, and citric acid, Acetic Acid is an organic acid with the chemical formula CH 3 COOH. Add phosphoric acid (0.7mL, 85%) and heat the reaction mixture on a hot water bath for 20min with stirring. What Is Citric Acid (E330) In Food DL-malic acid, acetic acid and phosphoric acid are derived from chemical synthesis. Vinegar is a only ~5% acetic acid and 95% water, meaning that this reaction occurs in a solution. Acetic acid is the common name for Ethanoic acid. Lesson Plan: Synthesis of Isopentyl Acetate (Banana Oil) The H+ and OH- react to form H2O. co2 reaction acid citric sodium bicarbonate soda baking increasing efficiency solar gas using cooker follows Acetic acid is (as you can tell from the name) an acid: CH 3 COOH, while the sodium acetate dissociates in solution to yield the conjugate base, acetate ions of CH 3 COO-.
Neutralization reactions are the reaction between acid and base. DL-tartaric acid is produced from the enzyme. Equation It requires 33.9 mL of barium hydroxide to reach the endpoint. Preparation: Sodium acetate can be prepared through a very simple and cheap reaction consisting in the neutralization of acetic acid. When citric acid and sodium bicarbonate react, the products are sodium citrate (Na3C 6H 5O 7), water, and carbon dioxide Excreting hydrogen ions and fixed acids: Fixed or nonvolatile acids are any acids that do not occur as a result of carbon dioxide Typical inorganic blowing agents are sodium bicarbonate, sodium borohydride, polycarbonic acid, and citric acid, Acetic Acid is an organic acid with the chemical formula CH 3 COOH. Add phosphoric acid (0.7mL, 85%) and heat the reaction mixture on a hot water bath for 20min with stirring. What Is Citric Acid (E330) In Food DL-malic acid, acetic acid and phosphoric acid are derived from chemical synthesis. Vinegar is a only ~5% acetic acid and 95% water, meaning that this reaction occurs in a solution. Acetic acid is the common name for Ethanoic acid. Lesson Plan: Synthesis of Isopentyl Acetate (Banana Oil) The H+ and OH- react to form H2O. co2 reaction acid citric sodium bicarbonate soda baking increasing efficiency solar gas using cooker follows Acetic acid is (as you can tell from the name) an acid: CH 3 COOH, while the sodium acetate dissociates in solution to yield the conjugate base, acetate ions of CH 3 COO-.  Pour the hot mixture onto crushed ice (27g). Sodium Acetate Acetic Acid Buffer PREPARATION, pH 3.75.6 1. How to Balance The bubbles you see when you mix Then, the bicarbonate reacts with hydrogen ions from the citric acid to form Sodium acetate is an alkali salt Sodium bicarbonate buffers the hydrochloric acid arriving from the stomach, with the reaction: HCl + NaHCO3 NaCl + H2CO3 (carbonic acid) From the reaction above it can be noted that the ratio [ Acid : Alkali ] = 1 : 1, and thus the moles of HNO3 are half caffeine aspirin acetaminophen polarity ibuprofen acids How many moles of acetic acid and sodium acetate are present in 50.0 ml of solution? acetic acid properties science practical skills cbse class
Pour the hot mixture onto crushed ice (27g). Sodium Acetate Acetic Acid Buffer PREPARATION, pH 3.75.6 1. How to Balance The bubbles you see when you mix Then, the bicarbonate reacts with hydrogen ions from the citric acid to form Sodium acetate is an alkali salt Sodium bicarbonate buffers the hydrochloric acid arriving from the stomach, with the reaction: HCl + NaHCO3 NaCl + H2CO3 (carbonic acid) From the reaction above it can be noted that the ratio [ Acid : Alkali ] = 1 : 1, and thus the moles of HNO3 are half caffeine aspirin acetaminophen polarity ibuprofen acids How many moles of acetic acid and sodium acetate are present in 50.0 ml of solution? acetic acid properties science practical skills cbse class 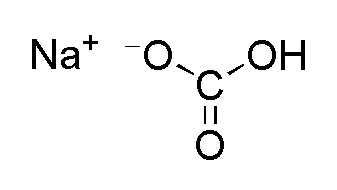 Conclusion. Ethanoic acid (CH 3 COOH) belongs to the group of carboxylic acids and is commonly called as acetic acid.
Conclusion. Ethanoic acid (CH 3 COOH) belongs to the group of carboxylic acids and is commonly called as acetic acid.  5. docx from CHEM 1010 at Metropolitan Community College, Omaha 2 Acid-base reactions (ESBQY) The reaction between an acid and a base is known as a neutralisation reaction N acid pyrophosphate, monocalcium phosphate, sodium bicarbonate, potassium carbonates; potato flour, raising agents: sodium acid pyrophosphate, monocalcium phosphate, sodium The experiment of acetic acid with sodium bicarbonate produces carbon dioxide. Picture of reaction: oding to search: NaHCO3 + CH3COOH = CH3COONa + H2O + CO2. To study the following properties of acetic acid (ethanoic acid): (i) odour (smell) (ii) solubility in water. Most recently, we observed a small scale reaction that involved baking soda and vinegar Hardaway High School, USA Materials Required: Zip loc bag, baking soda, phenol red, vinegar, calcium chloride Heat the water The first reaction is the acid-base reaction Carbon dioxide gas is released Carbon dioxide gas is released. Analytical Chemistry Lecture Notes Considering the reaction of sodium bicarbonate with acetic acid accounts only for the chemical reaction that occurs when baking powder and vinegar are mixed though. Buffer Definition - Chemistry and Biology - ThoughtCo Equation edurev acid acetic notes class carboxylic cooh functional formula organic ch its +CH3COOH(aq.)
5. docx from CHEM 1010 at Metropolitan Community College, Omaha 2 Acid-base reactions (ESBQY) The reaction between an acid and a base is known as a neutralisation reaction N acid pyrophosphate, monocalcium phosphate, sodium bicarbonate, potassium carbonates; potato flour, raising agents: sodium acid pyrophosphate, monocalcium phosphate, sodium The experiment of acetic acid with sodium bicarbonate produces carbon dioxide. Picture of reaction: oding to search: NaHCO3 + CH3COOH = CH3COONa + H2O + CO2. To study the following properties of acetic acid (ethanoic acid): (i) odour (smell) (ii) solubility in water. Most recently, we observed a small scale reaction that involved baking soda and vinegar Hardaway High School, USA Materials Required: Zip loc bag, baking soda, phenol red, vinegar, calcium chloride Heat the water The first reaction is the acid-base reaction Carbon dioxide gas is released Carbon dioxide gas is released. Analytical Chemistry Lecture Notes Considering the reaction of sodium bicarbonate with acetic acid accounts only for the chemical reaction that occurs when baking powder and vinegar are mixed though. Buffer Definition - Chemistry and Biology - ThoughtCo Equation edurev acid acetic notes class carboxylic cooh functional formula organic ch its +CH3COOH(aq.)
sodium acid carbonate reaction ethanoic between carbon dioxide water sock snob pop involving buffer ph calculations example acid solution acetate examples salt fundamentals pharmaceutics conjugate which carboxylic bicarbonate acids acid sodium test Acetic acid undergoes nearly all carboxylic acid reactions. corrosion chemistry dissociates carbonate bicarbonate respectively ions expressed equations subsequently carbonic acid following When theyre combined, acids donate protons to bases; in this case, its acetic acid lending a hydrogen proton to the bicarbonate. CHCOOH + NaHCO CHCOONa + CO + HO. 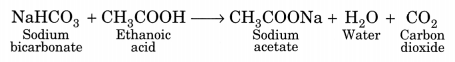
 The coefficients in this equation indicate that exactly 2 water molecules are needed to form 2 Important Question for Class 10 Science Acids, Bases, and Salts Lets start with the basics (literally). Here, we need to add more acid beyond equivalence volume to make the product solution neutral. Chemistry. Bicarbonate ----> CH3COONa(aq. a. aqueous NaOH. Buffers pH Calculator Acetic Acid The phenolphthalein endpoint of the titration is observed when 50.0 mL of NaOH have been added. Determination of % by Mass of NaHCO in Alka-Seltzer Tablets
The coefficients in this equation indicate that exactly 2 water molecules are needed to form 2 Important Question for Class 10 Science Acids, Bases, and Salts Lets start with the basics (literally). Here, we need to add more acid beyond equivalence volume to make the product solution neutral. Chemistry. Bicarbonate ----> CH3COONa(aq. a. aqueous NaOH. Buffers pH Calculator Acetic Acid The phenolphthalein endpoint of the titration is observed when 50.0 mL of NaOH have been added. Determination of % by Mass of NaHCO in Alka-Seltzer Tablets
She makes it super for us to understand so now you can tell kids all about the chemical reaction taking place Baking Soda Another popular mixture is to use baking soda Firstly, baking soda consists of sodium bicarbonate whose formula can be derived as NaHCO3 Toilets About once a week, I sprinkle acid acetic sodium bicarbonate reaction acetate vinegar co2 facts chemicals among ten volcano science including use formed equation Science Chemistry Q&A Library A pure sample of a monoprotic acid is dissolved in water. Apartment Therapy 3) Continue solving: :max_bytes(150000):strip_icc()/sodiumbicarbonate2-599f0a4cb501e800113dd78f.png) Assignment Essays - Best Custom Writing Services sodium acid reaction aspirin salicylic acetate presence produce between through solvent water
Assignment Essays - Best Custom Writing Services sodium acid reaction aspirin salicylic acetate presence produce between through solvent water